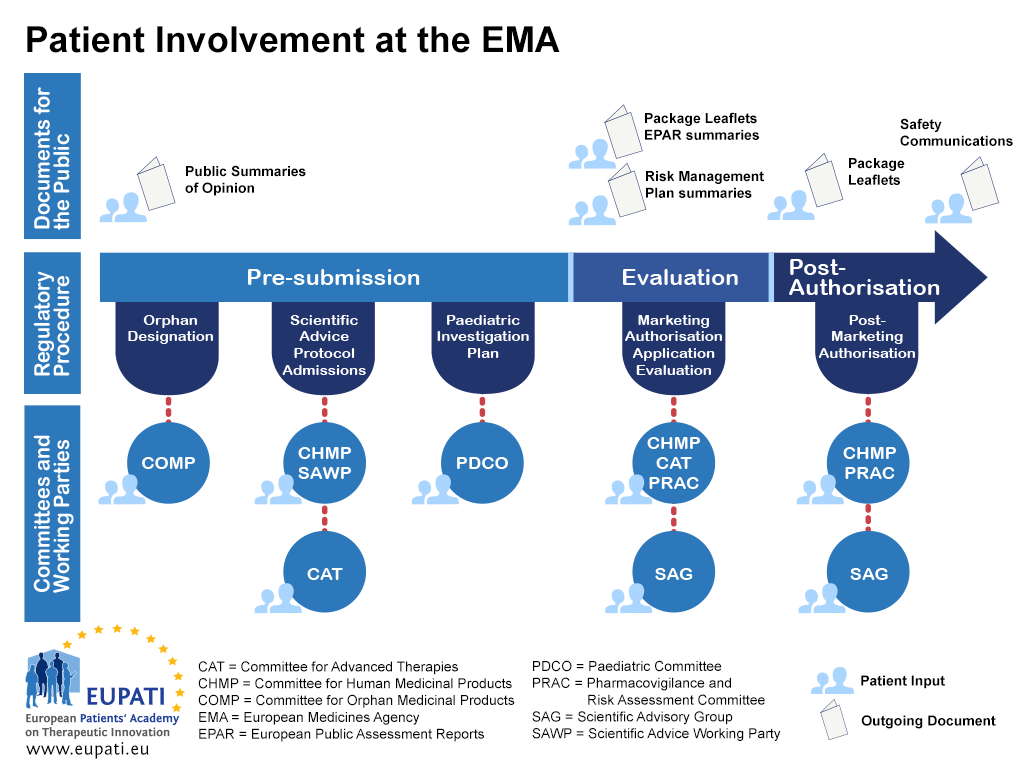
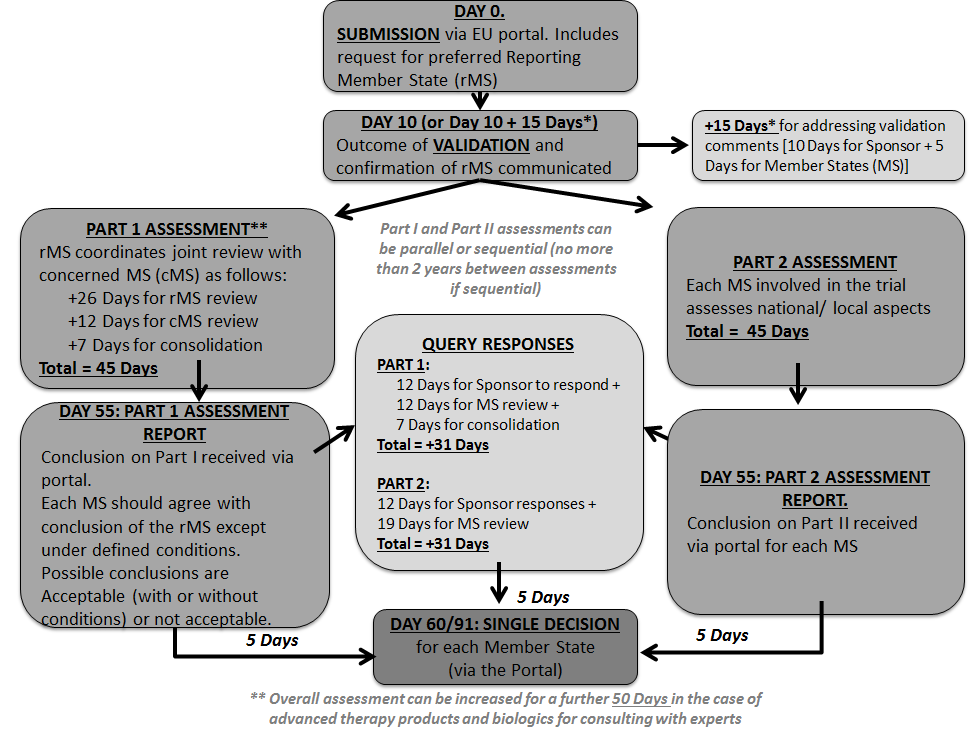
When innovation outpaces regulations: The legal challenges for direct‐to‐patient supply of investigational medicinal products - Malone - 2022 - British Journal of Clinical Pharmacology - Wiley Online Library

Comparison of power calculated as per the EMA guidelines among clinical... | Download Scientific Diagram

EMA & FDA Approvals and Recommendations in 2020 for Oncology Drugs and Diagnostics/Devices | CATO SMS
Guideline on the requirements to the chemical and pharmaceutical quality documentation concerning investigational medicinal prod

Frontiers | A Regulatory Risk-Based Approach to ATMP/CGT Development: Integrating Scientific Challenges With Current Regulatory Expectations